PRA number
Published date
Product description
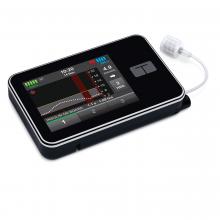
t:slim X2 Insulin Pump
The t:slim X2 Insulin Pump is intended for continuous insulin delivery and delivery of user activated insulin boluses, for the management of diabetes mellitus in persons requiring insulin.
Product code: 1006419
Software version of 6.4.1 or prior
ARTG 304681
(Australasian Medical & Scientific Ltd - Infusion pump, insulin, ambulatory)
Reasons the product is recalled
The insulin pump may experience software issues which may disrupt insulin delivery.
The hazards to consumers
The issues may result in under delivery of insulin that may cause hyperglycemia, which can lead to serious health complications.
In severe cases of hyperglycemia, the user may experience diabetic ketoacidosis and may require hospitalisation or intervention from a medical professional.
What consumers should do
Consumers can continue using the pump as described in the User Guide. It is advised to have an alternate means of insulin therapy available. Please update your pump to the latest software version at the earliest possible opportunity.
For more details, see https://apps.tga.gov.au/PROD/SARA/arn-detail.aspx?k=RC-2022-RN-00627-1
For further information, contact Australasian Medical & Scientific Ltd by calling 1300 851 056 or via email at diabetes@amsl.com.au.
Where the product was sold
Dates available for sale
Responsible regulator
Therapeutic Goods Administration is the responsible regulator for this recall.