Reason the product is recalled
The Therapeutic Goods Administration (TGA) has tested a product labelled Maxagra capsules and found that:
•the capsules contain the undeclared substance sildenafil; and
•the capsules contain the undeclared antibiotic substance oxytetracycline
•the capsules contain the undeclared substance sildenafil; and
•the capsules contain the undeclared antibiotic substance oxytetracycline
The hazards to consumers
Consumers are advised that sildenafil and oxytetracycline are both prescription-only substances in Australia.
The supply of Maxagra capsules containing sildenafil and oxytetracycline is illegal.
The supply of Maxagra capsules containing sildenafil and oxytetracycline is illegal.
What consumers should do
Consumers should stop taking Maxagra capsules and take any remaining capsules to your local pharmacy for safe disposal.
If you have any concerns arising from your use of this product, consult your health care practitioner.
If you have any concerns arising from your use of this product, consult your health care practitioner.
Details to help identify the product
Supplier running the recall
Cleanicall
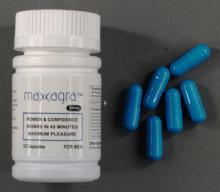
Identifying product features
All Maxagra capsules
Identifying numbers
Other identifying numbers
All Maxagra capsules
Responsible regulators
Regulators are established or appointed by government. They enforce regulations and rules.
Quote PRA number 2016/15225 when contacting a regulator about this recall.
Recall and remedy questions
Contact the Therapeutic Goods Administration if you have:
- a question about the remedy being offered to you by the business that is responsible for managing this recall, or
- concerns about the way the business is managing this recall.