Reason the product is recalled
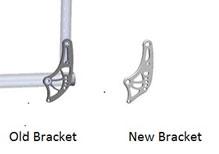
Over time, and under some usage conditions, the backrest brackets may break and detach from the chair frame.
The hazards to consumers
If a backrest bracket breaks and goes unnoticed by the user or care giver, there is a risk that the occupant could fall out of the seat and sustain injury to the head or lower back.
What consumers should do
If you or someone you provide care for uses a Quickie Q7 manual wheelchair manufactured between 1 September 2009 and 27 November 2013 (see above for affected model and serial numbers), be aware of this recall for product correction.
You will be contacted by the dealer who supplied your device to arrange replacement of affected backrest brackets.
If you have any questions or concerns about this issue, or if you think you have an affected device and are not contacted, talk to your dealer or contact Sunrise Medical by telephone on 02 9678 6600 or email enquiries@sunrisemedical.com.au
You will be contacted by the dealer who supplied your device to arrange replacement of affected backrest brackets.
If you have any questions or concerns about this issue, or if you think you have an affected device and are not contacted, talk to your dealer or contact Sunrise Medical by telephone on 02 9678 6600 or email enquiries@sunrisemedical.com.au
Details to help identify the product
Supplier running the recall
Sunrise Medical Pty Ltd
Identifying product features
Model number: EIR4
Serial number range: R4-007239 to R4-022394
Dates of manufacture: 1 September 2009 to 27 November 2013
Where the product was sold
Nationally
Responsible regulators
Regulators are established or appointed by government. They enforce regulations and rules.
Quote PRA number 2016/15593 when contacting a regulator about this recall.
Recall and remedy questions
Contact the Therapeutic Goods Administration if you have:
- a question about the remedy being offered to you by the business that is responsible for managing this recall, or
- concerns about the way the business is managing this recall.